Project Name: Salt Energy Battery
Your Name: Panharith Yav
Question:
What are the elements can be used to create the saltwater battery?
Background Information:
The electrical battery is the devices that made out of one or more electrochemical cell which is a device that necessary to generate electrical energy or facilitate the chemical reaction to generate the energy. Nowadays, batteries are really common daily in used for flashlights, phones, cameras and many other electrical suppliers. Moreover, there are two terminals on the batteries which are the positive terminal called cathode and the negative terminal called anode. With the electrolytes inside the battery, it allows the cathode and anode to exchange the ions when they move in one direction as they deliver the electricity and it will recharge when it moves to another direction. Currently, the batteries that people use daily are really effective to the environment when they throw it away. So, now people are trying to discover a new type of batteries that have no damage on the environment and sustainable for daily life uses.
Salt Water Battery:
Saltwater battery is the battery that has salty water, copper, zinc that attached to each other in the cup. With normal fresh water is very poor to the conductor of electricity but if we add salt into the solution it’ll become an electrolyte solution that can conduct electricity. So by adding salt to the pure water, when the salt or sodium chloride dissolved, they separate into different electrically charged atom which is called ions; when the salt is dissolved in will turn into the positive Na (sodium) ions and the negative Cl (chloride) ions. Additionally, electricity work through the negative charge and positive charge in the battery. Normally, we use copper wire in the electrical product; copper is a type of metal which it can conduct electricity really well. Zinc more readily to loses electrons than copper, so if we place zinc and copper metal in solutions of their salts can cause electrons to flow through an external wire which leads from the zinc to the copper. So in the middle of the solution, we can place a LED light to test, you will see the light is gleaming.
Hypothesis:
The zinc will lose their electrons to try to balance their atoms, so the copper will receive the electrons from zinc which in that process there is electricity between both of it.
Experiment or Design:
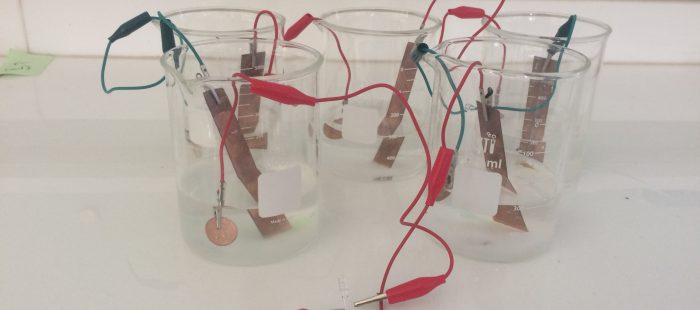
To design this experiment first we need five medium-size beakers, six alligators clips, five zinc coins and five copper plates.
Step to Build the Experiment:
Step 1: Filled the beakers with 200 mL of fresh water each.
Step 2: Add 3 tablespoons of table salt to those beakers.
Step 3: Connect the alligator clip one side to the zinc coins and another to the copper plate.
**(Make Sure there a clip connect to only zinc coin and another connection to only copper plate)**
Step 4: Place the coppers plates and zinc coin in the beakers (in one beaker there should be one zinc coin and one copper plate that not touch each other).
Step 5: There should be one beaker with the only copper plate and one with only zinc coin so place the remaining copper and zinc to the beaker where it missing.
Step 6: After placing the two remaining component there should be two sides of the alligators clip left.
Step 7: Connect the two side of the alligators clip to electronic devices such as LED and voltmeter to find how much the salt battery can conduct.
Data Charts:

The data show that when we added more salt into the water the voltage is increased at the same time.
Results:

According to the data above, in the result, the salt water battery is able to conduct a small amount of electricity. The graph shows that the voltage is increased over time when you add more salt to the water. Moreover, after the voltage is increasing it often falling back for example in about the 22 seconds the voltage is increasing but look at the 27 and 30 seconds the voltage is falling back. Even though this is a few voltages that the salt water battery had conducted, you can say that if you were to design it on a larger scale it may run for a whole house or classroom light. Additionally, if you look at the LED light that attached to the solution you can clearly saw that it actually glow. So when zinc loses electrons and the copper gain one, and if we place zinc and copper metal in solutions of their salts can cause electrons to flow through an external wire which leads from the zinc to the copper. The electron that flows from zinc to copper will create the current and will to be electricity.
Conclusion:
The graph result shows that the voltage is increased over time when you add more salt to the water. Moreover, after the voltage is increasing it often falling back for example in about the 22 seconds the voltage is increasing but look at the 27 and 30 seconds the voltage is falling back. This clearly shows that the salt water battery is working and we also saw the there are some amount of voltages. Moreover, we can see the pattern when the voltages were going up and falling back when and after we added more salt into the solution, According to the result and the data distributed we can say that the result strongly supports the hypothesis up above. Additionally, the result means that the topic of salt water battery should be the next focus for scientists who try to develop the green energy. Besides, this experiment is on a small scale so the result doesn’t really affect the people perspective on using the salt water battery but if it were to be a larger scale it would of do so. So the next plan is, how can we develop salt water battery into a larger scale and when are we able to do it so?
Bibliography:
Works Cited
“Battery (electricity).” Wikipedia. Wikimedia Foundation, 30 Aug. 2017. Web. 14 Sept. 2017.
Dhurde, Samir. “Salt Water Battery.” Toys from Trash. Web. 14 Sept. 2017.
“Electrochemical Cell.” Wikipedia. Wikimedia Foundation, 08 Sept. 2017. Web. 14 Sept. 2017.
“How Batteries Work.” HowStuffWorks. 01 Apr. 2000. Web. 14 Sept. 2017.
“How Salt Water Batteries Can Be Used for Safe, Clean Energy Storage.” How Salt Water Batteries Can Be Used for Safe, Clean Energy Storage – Electronic Products. Web. 14 Sept. 2017.
“Welcome to Battery University!” Basic to Advanced Battery Information from Battery University. Web. 14 Sept. 2017.
Book:
Hands-On Chemistry Activities with Real-Life Applications
3.4.2 Energy Transformation, Page 283